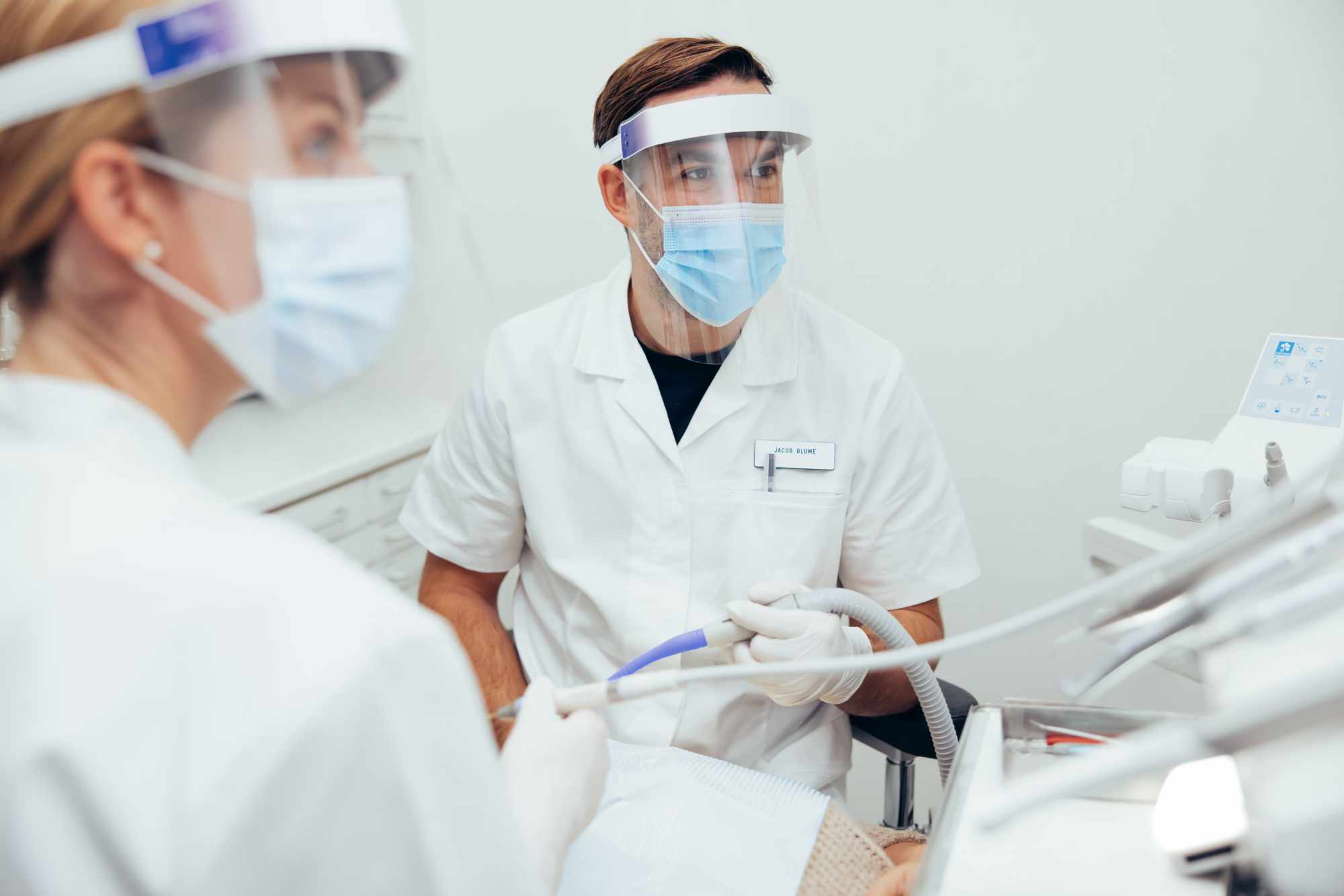
In close cooperation with industry and clinicians, MECEOR helps to develop product and process innovations in medical technology faster and more demand-driven.
The newly introduced Medical Device Regulation (MDR) of the European Union requires, among other things, significantly more clinical trials and leads to more complex approval procedures than through the previous MDD (Medical Device Directive), which can delay the market entry of new products of highly innovative SMEs (small and medium-sized enterprises) and start-ups.
These companies often lack the Know-How and experience regarding the correct planning, organization and evaluation of clinical trials as well as obtaining the ethics committee approval. Due to the high workload, physicians employed in clinics are less and less able to support these tasks with the required time efficiency or even to take them over entirely.
The MECEOR platform primarily aims to act as a catalyst for young companies, start-ups or SMEs in particular to ensure a high-quality and accelerated market entry of their innovations and technologies. This service and company idea is based on our many years of experience in the development and approval of medical products and technologies.
One, if not the decisive, factor for the quality of the support is the focus and specialization of the MECEOR platform on oral medicine. The medical technology used there is very familiar to the founders from their previous fields of work.
Our Services
Clinical Research – MDR (Medical Device Regulation)
MECEOR’s primary service is based on structuring, implementing, monitoring and scientifically evaluating preclinical and clinical research specifically for each client.
We provide our clients with clinical data in such a way that they can meet the approval requirements of the new MDR and bring their product or technology to market in a timely manner. MECEOR intervenes in a mediating, supporting and catalyzing way in the generation of (pre-)clinical research studies and results.
Consulting “Internal Process Improvement” and MDR Implementation
Companies that have already partially or fully implemented approval-relevant or regulatory affairs processes often lack clean and stringent internal processes, while start-ups often lack the necessary expertise to do so.
We advise these companies on optimizing and documenting the necessary internal processes in order to achieve efficient and effective compliance with the Medical Device Regulation MDR. MECEOR also provides direct support in the implementation of the new MDR, especially in the combination of MDR and clinical trials, and in the approval of your innovative medical devices.
Patient data
MECEOR provides anonymized and validated patient data for artificial intelligence (AI)-using software development and tele-medicine procedures.
MECEOR not only accesses existing patient data from Frankfurt University Hospital, but also uses the dental office on campus and its associated research and teaching practices in Germany as sources. A network of over 800 master’s students from more than 70 countries will also contribute patient data from their dental offices and, in some cases, large dental clinics upon request. MECEOR and its associated network have a very large data pool from which the necessary data can be processed and validated for projects.
Intermediation of capital providers
MECEOR arranges capital for young companies and SMEs to finance projects, especially in connection with clinical trials.
This makes medical product development through the always cost-intensive clinical research possible and fundable for some of these companies in the first place. With MECEOR’s support, the probability of attracting a potential investor to finance a medical product increases significantly.
Marketing and commercialization
MECEOR supports the successful commercialization of clinically tested and approved products.
SMEs or start-ups usually have no or only insufficiently developed commercial distribution structures or networks. Here MECEOR can mediate distributors in oral medicine through its network and partners and open up distribution channels. Alternatively, there is also the possibility of establishing contact with capital providers, who can in turn finance the companies’ own direct, but initially capital-intensive, distribution channel.
Our expertise combined with an existing extremely
broad network in oral medicine, are the basis for a comprehensive, long-term and sustainable support by MECEOR in the implementation of your innovation.
Advantages for you and your company
MECEOR’s service and business idea is based on the long experience of the company’s founders and partners in the development of medical technologies with over 100 years of combined experience in oral medicine.
MECEOR is focused and specialized in oral medicine.
The medical technology used there is very familiar to the founders and partners and there is already a very broad and global network in this market segment.
MECEOR helps companies bring their oral medical technology innovations to market faster.
For start-ups and SMEs, this means high performance in the development, approval and market launch of their innovative products. Established companies, where capacity bottlenecks arise in the approval procedure due to the extended requirements of the MDR, can realize early sales with their innovative product through the support of MECEOR.
MECEOR serves the rapidly increasing demand for validated anonymized patient data.
For the development of AI-based software solutions and/or for the optimization of tele-medicine, MECEOR provides anonymized and validated patient data.
Due to our company location in affiliation to the campus of the University Hospital Frankfurt/M with associated dental clinic, this facilitates and enables our customers to have full access to a clinical environment. Furthermore, this practical environment reduces the risk of erroneous developments and increases the quality of innovations.
Together with the offer of venture capital mediation, we help innovative companies in the field of oral medicine to quickly enter the market.
MECEOR is an industry-in-clinic platform,
focused and specialized in oral medicine. We improve collaborations between industry and clinics.
What does MECEOR stand for?
The company name or acronym MECEOR is derived from the three words medicine, CERN and oral.
MECEOR is a company that is exclusively active in ORAL MEDICINE and catalyzes and accelerates (oral) medical product approval processes. CERN stands symbolically for acceleration (CERN = known to be the largest proton accelerator worldwide – generally a symbol for acceleration).
Why MECEOR?
The trigger and primary reason for founding MECEOR was the newly introduced Medical Device Regulation (MDR) of the European Union.
The new MDR inevitably leads to a very complex and extremely time-consuming approval procedure for medical devices. First and foremost, it requires significantly more clinical trials before a product can be approved and marketed.
However, young companies in particular often lack the knowledge and experience required for the approval of their highly innovative products and ideas, the correct planning, organization and evaluation of clinical studies, and even the obtaining of an ethics committee approval.
Very good and innovative product ideas and companies often fail due to the complex approval formalities. In the best case, the market entry of a new medical product is achieved only after a considerable delay.
That’s why MECEOR!
MECEOR starts exactly at this point.
MECEOR has exactly the core competencies that SMEs and start-ups often lack to get their medical device ideas approved.
MECEOR acts as an approval catalyst for companies, start-ups and SMEs to achieve an accelerated market entry of new Med-Tech products and innovative technologies.
For established companies, MECEOR supports the efficient implementation of MDR requirements and helps to eliminate capacity bottlenecks.
The minds behind MECEOR
MECEOR’s service and business idea is based on the many years of experience of the company’s founders and partners in the development of medical technologies, with over 100 years of combined experience in oral medicine.

Dipl. Ing. Cornelius Geist

Dr. Paul Martin Weigl, PhD
MECEOR
We would be pleased to inform you about our
services about the approval of your medical device.
| SITE | MECEOR GmbH Altenhoeferallee 3 60438 Frankfurt am Main Germany |
| Phone | +49 (0) 69 3486 8064 0 |
| office@meceor.de | |
| Web | www.meceor.de |
